When people think of treatments for cancer they likely think first of drugs, both traditional chemotherapy and, more recently, targeted drugs. Increasingly, however, cancer therapies may be vaccines, antibodies, or even whole cells. Cell therapy is a relatively new but rapidly growing area of research, and one where Memorial Sloan Kettering Cancer Center is taking a lead in initiating new clinical trials and developing innovative treatments for patients.
“We are at a critical time when a number of cell therapies are starting to demonstrate some therapeutic benefits,” says Isabelle Rivière, Director of Memorial Sloan Kettering’s newly established Cell Therapy and Cell Engineering Facility (CTCEF). “There are still a number of hurdles to overcome from the processing and manufacturing point of view, but we are now at a stage where the scientific and medical communities believe it’s worthwhile to move forward and test these new treatments.”
Memorial Sloan Kettering’s efforts to develop cell-based treatments have been largely initiated by Michel Sadelain, who directs the Gene Transfer and Gene Expression Laboratory and the Center for Cell Engineering (CCE). The CCE is a collaborative partnership between physicians and scientists that brings together researchers who investigate areas that span stem cell and immune cell engineering, cell delivery and stem cell transplantation, directed cell differentiation, gene transfer, and gene repair.
The term cell engineering encompasses several different processes: cell purification (separating one cell type from other types), cell expansion (inducing cells to divide and reproduce in order to create larger quantities), cell differentiation (exposing cells to factors that stimulate them to develop into a more specialized cell type), and gene transfer (inserting new or modified genes into cells to change their behavior). Cell therapies can be created by engineering a patient’s own cells or by engineering cells from a donor.
“The purpose for harnessing cell engineering to develop effective cell therapies is ambitious,” Dr. Sadelain says. “Can cancer be effectively treated with a single infusion of engineered immune cells that persist in the patient for as long as they are needed to halt tumor progression? Can long-term production of healthy blood cells be generated in patients treated with genetically modified or expanded stem cells? These questions, applied to blood cell types and others, such as neurons and endocrine cells, are being investigated in our CCE.”
Targeted immunotherapy
One type of cell therapy that has been pioneered by Memorial Sloan Kettering is called targeted immunotherapy. Targeted immunotherapy aims to specifically instruct the immune system to recognize and attack tumor cells. Medical oncologist Renier Brentjens, along with Dr. Sadelain and other colleagues, has designed clinical trials that are currently under way for patients with advanced chronic lymphocytic leukemia (CLL) and B cell acute lymphoblastic leukemia (ALL), two forms of blood cancer.

Transplant specialist Farid Boulad plans a clinical trial in which stem cells are removed, modified, and then returned to the same patient.
The protocol involves isolating white blood cells called T cells from patients and introducing a new gene into the cells. The gene encodes a receptor that enables the T cells to recognize a protein called CD19, which is present in the leukemia cells. After the gene is transferred and expressed, the T cells are infused back to the patient where they seek out and attack the patient’s cancer, inducing a variety of different immunologic reactions that contribute to tumor eradication.
The genes are introduced into the T cell using an engineered vector. Vectors are disabled viruses that cannot replicate but efficiently shuttle their genetic cargo into the host cell.
“Over the past ten years we have been a leading center in developing this novel technology in the laboratory, and we were the first center to bring this CD19-targeted approach to the clinic,” Dr. Brentjens explains. “We have refined the design of the artificial receptors [termed chimeric antigen receptors, or CARs] to increase the potency of T cells, relying on extensive studies performed in animal models to assess the effectiveness of the genetically targeted T cells,” he says, noting that much of the early research was supported by Memorial Sloan Kettering’s Experimental Therapeutics Center.
A report by Drs. Brentjens, Rivière, and Sadelain, along with oncologists from Memorial Sloan Kettering’s Leukemia Service, published in Blood in August 2011 described phase 1 studies in which the therapy effectively killed tumor cells or controlled tumor growth in four out of nine patients treated, with few side effects. Another study published by the University of Pennsylvania this summer also showed promising results using engineered T cells to treat CLL. “This is an exciting time for us,” Dr. Brentjens adds. “The recent case reports from several institutions showing efficacy with targeted T cells are extremely encouraging. While much more work still needs to be done to optimize this therapy, there are significant, objective reasons for enthusiasm.”
Memorial Sloan Kettering pediatric oncologist Nancy A. Kernan recently launched a similar trial for pediatric patients with ALL who have relapsed after receiving bone marrow transplants. The protocol uses donor T cells that have been engineered to treat post-transplant Epstein-Barr virus infections, which are then genetically targeted to CD19 using the same vector as in Dr. Brentjens’s study.

Genitourinary oncologist Susan Slovin has promising results from a prostate cancer clinical trial that makes use of engineered cells.
Another area in which targeted immunotherapy has been brought to the clinic is prostate cancer. Genitourinary oncologist Susan F. Slovin has been collaborating with Drs. Sadelain and Rivière on a T cell therapy targeting a protein called prostate-specific membrane antigen (PSMA). As prostate cancer progresses and becomes resistant to hormone treatment, the expression of PSMA increases a thousandfold. A current trial is for patients who have metastatic cancer and have failed hormonal therapy and at least one prior chemotherapy. Patients are given a single dose of chemotherapy to temporarily remove host cells that could interfere with the function of the infused T cells.
“We have strong preclinical models that support the technology, showing not only that these engineered cells cause an antitumor effect but that they migrate to the tumor once they are in the body,” Dr. Slovin says. “We’ve treated three patients so far and the results are promising. This treatment affords patients the opportunity to try something that has the potential to change the biology of the disease — and by that I mean slowing the disease’s progression — with little adverse tolerance.”
Two additional trials using targeted immunotherapy are currently in late stages of preclinical development at Memorial Sloan Kettering. Surgeon Prasad S. Adusumilli is working on a cell therapy to treat mesothelioma, a rare tumor of the chest cavity. The treatment will employ T cells engineered to target mesothelin, a cell-surface protein that is overexpressed in 80 percent of mesothelioma patients and the presence of which can be determined with a simple blood test. His team has completed the efficacy and safety studies and is planning to initiate a clinical trial in 2012, using the engineered T cells in combination with chemotherapy.
Medical oncologist and immunologist Jedd D. Wolchok is designing a cell-based treatment for melanoma, a deadly form of skin cancer. In this therapy, T cells are engineered to recognize and kill cells producing the protein NY-ESO-1, a therapeutic target discovered at Memorial Sloan Kettering and found in melanoma and other cancers. Patient enrollment in the trial is expected to begin in 2013.
Back to topEngineering stem cells
Stem cells are the other major focus of investigation in the CCE. Dr. Sadelain and his colleagues have been working on a cell-based therapy for beta(β)-thalassemia, an inherited blood disorder found in people of Mediterranean, Asian, or African descent. The disease is characterized by the inability of red blood cells to make a protein called β-globin. In a related disorder affecting the same gene, sickle cell disease, the affected patients make an abnormal form of β-globin. The β-globin protein is part of hemoglobin, a vital protein that delivers oxygen throughout the body.
Dr. Sadelain’s laboratory discovered a decade ago that β-thalassemia could be cured in mice by engineering hematopoietic stem cells — the blood-forming cells found in the bone marrow — to restore their ability to generate healthy red blood cells. Studies in mouse models of thalassemia showed that the engineered stem cells permanently established themselves and continued to generate red blood cells containing high levels of the β-globin protein years after the treatment was administered. The results suggested that patients suffering from β-thalassemia could be treated using this approach to offset their need for lifelong red blood cell transfusions.
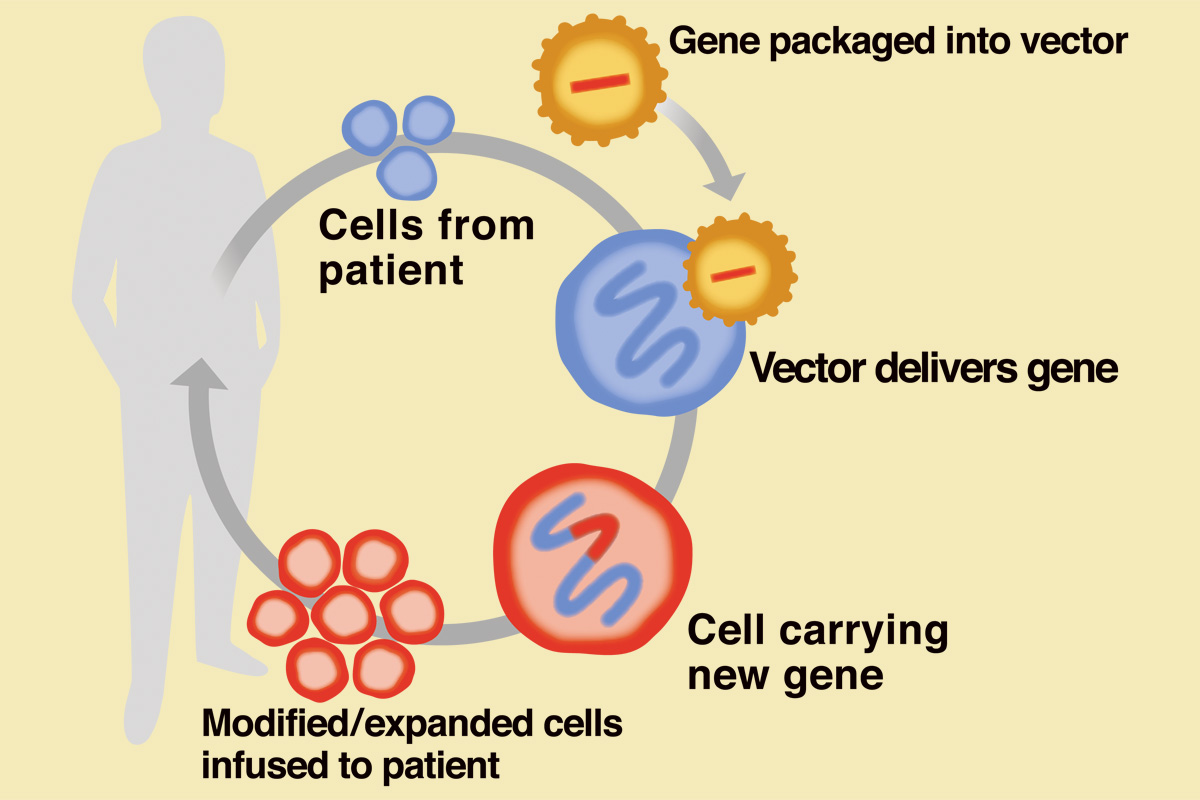
In cell engineering therapy, cells are removed from a patient or a healthy donor and genetically modified to enhance the cells’ characteristics and therapeutic potential. In most instances, the cells are expanded to yield adequate doses before being administered to the patient.
Following the demonstration by the CTCEF that the vector can be successfully delivered to human hematopoietic stem cells, a clinical team led by transplant specialist Farid Boulad is preparing to launch a clinical trial in which patients’ own stem cells will be removed, modified to contain the functional globin gene, and given back to the patients. The hematopoietic stem cells can come from the bone marrow or be extracted from peripheral blood. A low dose of chemotherapy will be given to suppress the body’s natural production of blood cells prior to the reinfusion of the stem cells engineered with the normal globin gene.
“The current treatment for these patients is a full bone marrow or stem cell transplant. But for patients who don’t have a matched donor, the only option now is regular blood transfusions, for life,” Dr. Boulad explains. “We have been doing transplants for β-thalassemia at Memorial Sloan Kettering since the 1980s and based on that experience, combined with Dr. Sadelain’s work, we decided to go forward with this new protocol.” Dr. Boulad is awaiting approval from the US Food and Drug Administration to begin the trial.
Another area of Memorial Sloan Kettering’s stem cell engineering research relates to expanding the potential use of umbilical cord blood. Cord blood is collected from the umbilical cords and placentas of healthy newborn babies. It is a rich source of blood-forming stem cells and is useful due to its rapid availability and the lesser requirement for a match between the patient and donor due to the less developed neonatal immune system. A team led by Juliet N. Barker, head of the Cord Blood Transplantation Program, has proven that cord blood transplantation can extend transplant access to patients from racial and ethnic minorities who lack other suitable donors and that cord blood transplantation yields survival rates comparable to those after transplantation using adult donors.
The use of cord blood can sometimes be problematic, however, because the number of cells collected from the donor may be insufficient to safely perform the transplant. A low dose of cord blood cells can delay the patient’s recovery, leading to prolonged hospitalization and other serious complications. Therefore, Dr. Barker, along with Sergio A. Giralt, the Adult Bone Marrow Transplant Service Chief, and other members of the CCE and CTCEF, is working to develop processes for expanding stem cells from cord blood in the laboratory. If these methods are successful, the results of cord blood transplantation could be greatly improved, making it possible to treat more patients.
Back to topExpanding facilities
Cell expansion methods are essential for improving several cell therapies. Cells that have undergone gene transfer, such as those created for targeted immunotherapy or for β-thalassemia treatment, can also be in short supply. The ability to safely generate larger supplies of cells for infusion to patients will improve the likelihood that these treatments will succeed and become more available.
Key to the growth of Memorial Sloan Kettering’s cell engineering capabilities has been the establishment of facilities based on good manufacturing practices (GMP) — the systems and practices that are required to be in place for the manufacture of pharmaceuticals. The Center’s cell engineering facilities are similar to an operating room, where airflow is filtered and controlled. The specialized staff working in the CTCEF wear protective suits, including hoods and facemasks.

Technicians in Memorial Sloan Kettering’s cell engineering facilities follow good manufacturing practices to protect patients’ cells from contamination.
“Depending on the protocol and the kind of processing the cells need, the cells prepared for infusion to patients may be in culture for days or weeks,” Dr. Rivière explains. “One therefore needs a very controlled environment to protect the cells and exclude any contamination. There are at present few GMP facilities around the country that can develop and implement cell engineering for the cell therapies of the future. But their number is growing.”
Memorial Sloan Kettering is now in the process of constructing new cell engineering facilities, which will be housed on the top floor in the second phase of the Zuckerman Research Center, currently being built on East 68th Street and due for completion in early 2014. The new facility will feature eight cell processing rooms, two vector production rooms, and one cell purification room as well as supporting areas, doubling the current amount of space.
“Another strength of our program is that we engineer most of our vectors in-house, instead of outsourcing them,” Dr. Rivière adds. “Having the ability to manufacture them ourselves speeds up the process, and is one of the reasons we were among the first institutions to start clinical trials in this area.”
“It’s no accident that Memorial Sloan Kettering is a leader in this field,” Dr. Sadelain concludes. “The research plays on the strengths of both the Sloan Kettering Institute and Memorial Hospital, where transplantation and transfusion medicines are solidly implanted. With the addition of the GMP laboratories, we have the three components — innovative research, clinical excellence, and cell engineering expertise — that are needed to advance this field. It positions us among a select group of institutions that can truly design, develop, and test novel concepts and methodologies for the new cell therapies and regenerative medicine.”
Back to top



